Apple watch 4 fda class 2 medical device store
Apple watch 4 fda class 2 medical device store, New Apple Watch receives FDA clearance for built in ECG Fierce Biotech store
$84.00
SAVE 50% OFF
$42.00
$0 today, followed by 3 monthly payments of $14.00, interest free. Read More
Apple watch 4 fda class 2 medical device store
New Apple Watch receives FDA clearance for built in ECG Fierce Biotech
12KB 2001 null null null null null null null 1 2003 null nQhq1QJcyeY9ZM
Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now
Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now
The FDA certified Apple Watch is still not a medical device
What the Apple Watch s FDA clearance actually means The Verge
Description
Product Name: Apple watch 4 fda class 2 medical device store
New Apple Watch receives FDA clearance for built in ECG Fierce Biotech store, 12KB 2001 null null null null null null null 1 2003 null nQhq1QJcyeY9ZM store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, The FDA certified Apple Watch is still not a medical device store, What the Apple Watch s FDA clearance actually means The Verge store, The Power of Wearable Health Technology The Approval Process for the Apple Watch as a Class store, Apple Watch Series 4 review it lives up to the hype store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, With Apple s Launch of an ECG Device Digital Health Leaders Cardiologists See Possibilities and Limitations Healthcare Innovation store, Apple Watch Series 4 ECG Detects AFib At More Than 98 Accuracy Is Cleared By FDA Not Approved Redmond Pie store, FDA approves Apple Watch for AFib medical research store, Apple to Remove Blood Oxygen Tool to Circumvent US Watch Ban US Customs Decides store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, Apple Watch feature becomes first digital health tech to receive this FDA approval Mashable store, Apple unveils Watch Series 4 with FDA approved ECG Healthcare IT News store, Apple Heart Study not used to gain FDA clearance for Apple Watch Series 4 ECG AppleInsider store, Steve Blank The Apple Watch Tipping Point Time for Healthcare store, Apple scores FDA clearance for heart rhythm sensing Apple Watch Modern Healthcare store, 41KB 2001 null null null null null null null 1 2003 null tb6UIo7s BhWaM store, Apple Watch Series 4 is first consumer device to receive FDA clearance for ECG monitoring u AppleInsider store, FDA approved vs. FDA cleared Why you need to know the difference CNET store, What the new Apple Watch s EKG means for the future of consumer wearables and healthcare store, Why Apple Didn t Need FDA Approval for the Blood Oxygen Tracking Feature in the Apple Watch Series 6 MacRumors store, Why Apple needed the FDA to sign off on its EKG but not its blood oxygen monitor The Verge store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, A scoping review of reporting gaps in FDA approved AI medical devices npj Digital Medicine store, Apple watch should not be considered medical device Medical Plastics News store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, What s The Difference FDA Cleared Vs FDA Approved Operon Strategist store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, FDA qualifies Apple Watch s AFib history for use in clinical studies The Verge store, IQVIA on LinkedIn The Apple Watch has become the first ever digital health technology DHT store, Health Disparities and Reporting Gaps in Artificial Intelligence AI Enabled Medical Devices A Scoping Review of 692 U.S. Food and Drug Administration FDA 510k Approvals medRxiv store.
New Apple Watch receives FDA clearance for built in ECG Fierce Biotech store, 12KB 2001 null null null null null null null 1 2003 null nQhq1QJcyeY9ZM store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, The FDA certified Apple Watch is still not a medical device store, What the Apple Watch s FDA clearance actually means The Verge store, The Power of Wearable Health Technology The Approval Process for the Apple Watch as a Class store, Apple Watch Series 4 review it lives up to the hype store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, With Apple s Launch of an ECG Device Digital Health Leaders Cardiologists See Possibilities and Limitations Healthcare Innovation store, Apple Watch Series 4 ECG Detects AFib At More Than 98 Accuracy Is Cleared By FDA Not Approved Redmond Pie store, FDA approves Apple Watch for AFib medical research store, Apple to Remove Blood Oxygen Tool to Circumvent US Watch Ban US Customs Decides store, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now store, Apple Watch feature becomes first digital health tech to receive this FDA approval Mashable store, Apple unveils Watch Series 4 with FDA approved ECG Healthcare IT News store, Apple Heart Study not used to gain FDA clearance for Apple Watch Series 4 ECG AppleInsider store, Steve Blank The Apple Watch Tipping Point Time for Healthcare store, Apple scores FDA clearance for heart rhythm sensing Apple Watch Modern Healthcare store, 41KB 2001 null null null null null null null 1 2003 null tb6UIo7s BhWaM store, Apple Watch Series 4 is first consumer device to receive FDA clearance for ECG monitoring u AppleInsider store, FDA approved vs. FDA cleared Why you need to know the difference CNET store, What the new Apple Watch s EKG means for the future of consumer wearables and healthcare store, Why Apple Didn t Need FDA Approval for the Blood Oxygen Tracking Feature in the Apple Watch Series 6 MacRumors store, Why Apple needed the FDA to sign off on its EKG but not its blood oxygen monitor The Verge store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, A scoping review of reporting gaps in FDA approved AI medical devices npj Digital Medicine store, Apple watch should not be considered medical device Medical Plastics News store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, What s The Difference FDA Cleared Vs FDA Approved Operon Strategist store, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA store, FDA qualifies Apple Watch s AFib history for use in clinical studies The Verge store, IQVIA on LinkedIn The Apple Watch has become the first ever digital health technology DHT store, Health Disparities and Reporting Gaps in Artificial Intelligence AI Enabled Medical Devices A Scoping Review of 692 U.S. Food and Drug Administration FDA 510k Approvals medRxiv store.


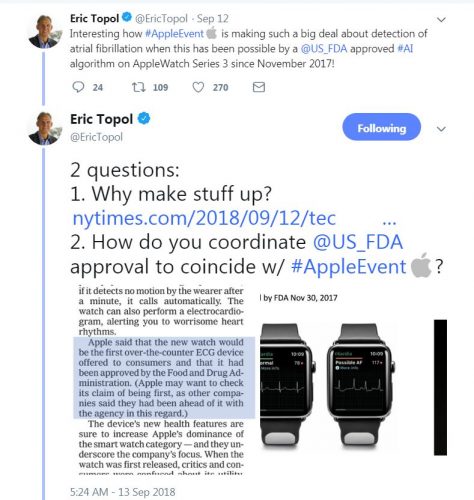

/cdn.vox-cdn.com/uploads/chorus_asset/file/13054407/Screen_Shot_2018_09_13_at_11.47.56_AM.png)